We know what it's like.
We’ve stood in the OR, answered the 6 a.m. calls, chased down info, faced critical decisions with incomplete data—and we built OpenSurg to solve the problems that kept coming back. Our tools are born from real surgical life, not a software lab.
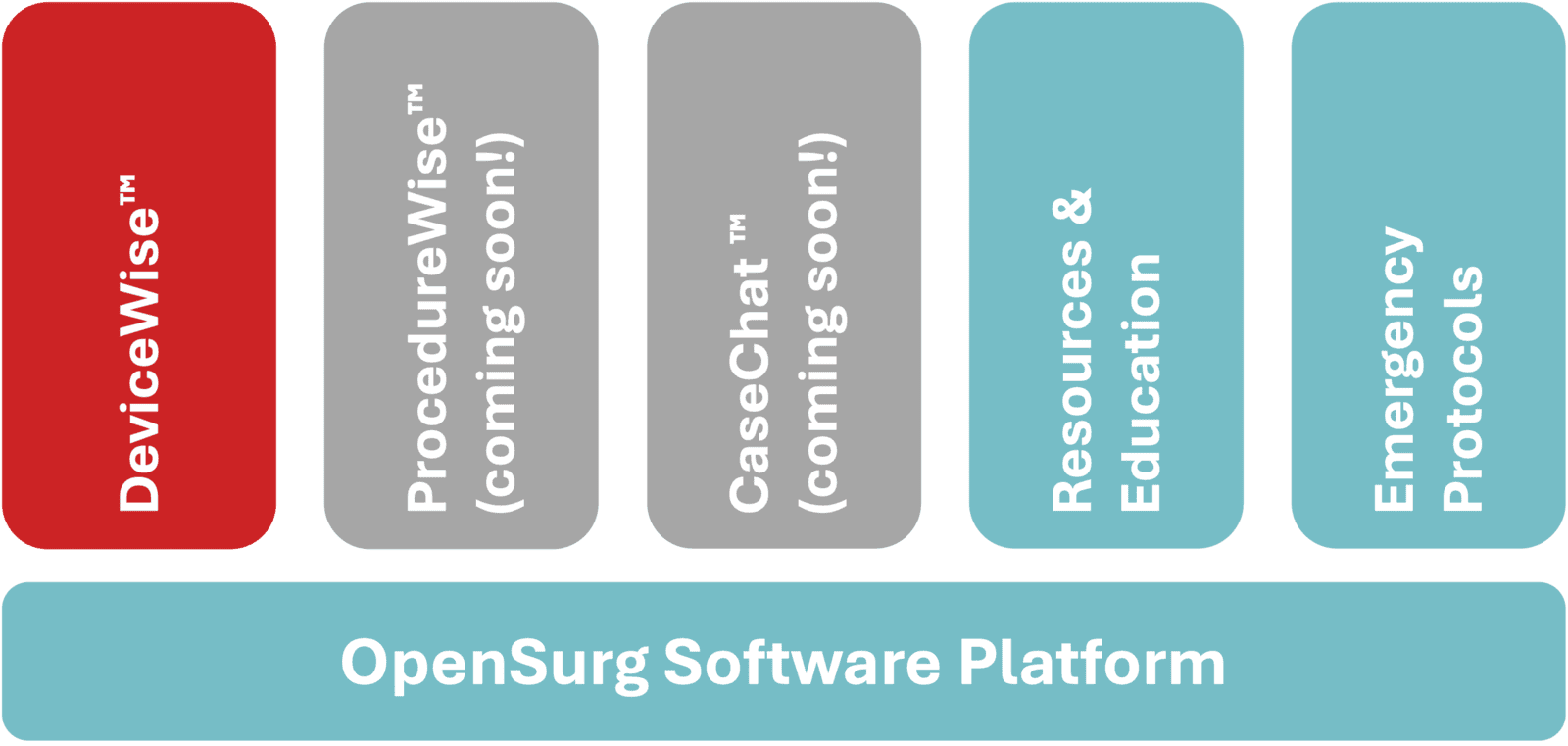
Manage implant risks with DeviceWise™
With the prevalence of active implanted medical devices increasing rapidly, mitigating surgery risks is more critical than ever.
